People are often confused about what Richard Bertram, a professor in the FSU Mathematics department and the director of the Biomathematics program, does for a living. At mathematics conferences he encounters people who thought he was a biologist, while at biology conferences he is known as “the math guy”. This is to be expected when you work at the interface of two disciplines, as Bertram has done since his days as a graduate student at Florida State University. Bertram became interested in the field after reading elegant work by John Rinzel that used a sophisticated mathematical technique called fast-slow decomposition to understand the nonlinear dynamics underlying bursting patterns in the electrical activity of neurons. After completing a dissertation on bursting oscillations under the supervision of Jerry Magnan (FSU Mathematics), Bertram moved to the National Institutes of Health as a postdoctoral fellow at the Mathematical Research Branch, where Rinzel was the lab chief. It was at the NIH that two of the most important events in Bertram's career took place: he began working with Arthur Sherman and he began working on pancreatic islets. Today, after many years and moves from Washington D.C., to Pennsylvania, and then back to Tallahassee, Bertram still works with Sherman on pancreatic islets, and other biological and mathematical topics. Along the way, he has picked up many other collaborators (98 co-authors so far), and even an experimental lab of his own at the Biomedical Research Facility at FSU. He has also branched out to several other topics in biology, chemistry, and dynamical systems theory.
The application areas of Bertram's current research focus are neuroscience, endocrinology, and the intersection of the two called neuroendocrinology. He develops mathematical models of the various systems, uses mathematical and computational techniques to analyze the models, develops predictions that will help support the model (or overturn it if the predictions fail), and then works with others to test the predictions in the laboratory. Within neuroscience, he collaborates with a team of scientists at FSU to understand how the neural circuitry in the bird brain develops so as to produce a highly stereotyped song, as occurs in the male zebra finch. This may seem to be a strange topic, and always gets the eye-rolling response when he mentions it at parties, but there is method to this madness. There are no primates that learn to vocalize by listening to adults, as humans do when learning to speak. The closest you can get to human vocalization is the male zebra finch, which learns its song by listening to and mimicking its father. So one good way to understand how humans acquire speech is to study the zebra finch. Bertram started working on this in 2006, when he was approached by Frank Johnson (FSU Psychology) to help solve a problem that came up when doing an acoustic analysis of the notes of the bird song. It turned out to be a statistics problem, so Bertram asked Wei Wu (FSU Statistics), who he had recently met during Wu's job interview, to join the group. The team has recently grown to include Rick Hyson (FSU Psychology), who is an expert at making electrical recordings from neurons in a brain slice. Bertram's role is to help in the development of analysis techniques for the bird song, and to develop mathematical models of the neural circuitry that underlies the vocalization. The team is funded by the National Science Foundation, and includes graduate students from Neuroscience and Mathematics.

Within the area of endocrinology, Bertram studies the biophysical mechanism of pulsatile insulin secretion from cells in the pancreas called β-cells. Malfunction in insulin secretion leads to type II diabetes, which is by far the most prevalent form of the disease. One sign of type II diabetes is the loss of insulin oscillations in the blood. Since his days as a postdoc at the NIH Bertram has studied how islets produce oscillations, and how the oscillations from ~1 million islets synchronize to give coherent insulin oscillations in the blood. This involves development of models and use of the fast-slow analysis technique to understand them. Much of that work has been with his long-time theoretical collaborator Arthur Sherman (NIH) and experimental collaborator Les Satin (Univ. Michigan). More recently, Bertram has begun collaborating with Mike Roper (FSU Chemistry) to design a very small device (called a microfluidic device) that can be used to test a theory on islet synchronization that can't be tested in the whole animal. Bertram has worked with students from Physics, Mathematics, and Chemistry on the islet research, which has been funded by the NSF, the American Heart Association, and most recently the NIH (with Roper as the lead investigator).
An area of the brain called the hypothalamus, and the nearby pituitary gland, work together to orchestrate hormone release from all the endocrine glands of the body, such as the adrenal gland, ovaries, testes, pineal gland, and others. In 2005 Bertram began a collaboration with Marc Freeman (FSU Biological Science, retired) to study rhythms that occur in pituitary hormone release. This research is funded by the NIH, which provides sufficient financial support for a fairly large group of mathematicians, physicists, and biologists to study the problem from many angles. When Freeman retired Bertram became the principal investigator of the lab, which he now runs in collaboration with two FSU research professors, Joel Tabak and Arturo Gonzalez-Iglesias (both FSU Mathematics). This is the only neuroendocrine lab in the world that combines in vivo (whole animal) studies, in vitro (dispersed pituitary cells and brain slice) studies, and mathematical modeling. Since Bertram has been part of the lab, they have worked with students from Mathematics, Biology, and Neuroscience, as well as several postdoctoral fellows.
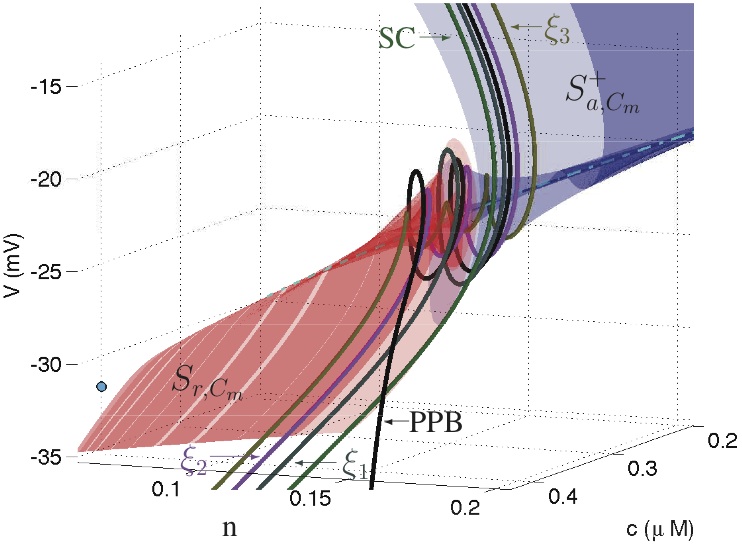
While Bertram's research is driven mostly by biological questions, these often lead to some very interesting mathematical questions. Most recently, Bertram has been studying mixed-mode oscillations, which consist of small and fast oscillations interrupted periodically by large excursions. Oscillations of this type occur in many pituitary cells and in single pancreatic β-cells, but although models developed by Bertram's lab easily reproduce the oscillations, understanding the dynamics that underly them is not so easy. A chance meeting with Martin Wechselberger (Univ. Sydney) at a conference in England in 2009, one of the leading theorists on mixed-mode oscillations, led to a collaboration that has revolved around abstract structures such as canard trajectories, folded-node singularities, and singular funnels. Bertram's team is now applying what they have learned from this mathematical analysis to laboratory experiments, using software that allows them to inject ionic currents described by mathematical models into real pituitary cells. These models are calibrated to the cell's behavior using the parallel processing capabilities of a programmable Graphics Processing Unit in a desktop computer, and then predictions are tested on the same cell used to calibrate the model. The mathematics again returns to the biology...